
COVID-19 update: July 8, 2022
CDC’s interim recommendations for Moderna and Pfizer-BioNTech vaccines for children from 6 months of age; FDA recommends Omicron BA.4/5 component for vaccine boosters

CDC’s interim recommendations for Moderna and Pfizer-BioNTech vaccines for children from 6 months of age; FDA recommends Omicron BA.4/5 component for vaccine boosters

One-year monitoring report shows increases in kidney transplants for Black, Hispanic, Asian and pediatric patients following policy changes.

Effective July 1, testing no longer required for HIV, HBV and HCV at the time of hospital admission for transplant candidates younger than age 12.

Starting late summer 2022, any caller who requests access to private information or UNet services from UNOS, will use a new identity verification process that utilizes Authy, the same application used for accessing UNet.

Transplant hospitals that have completed the 2021 UNOS Staffing Survey may now access their program’s results, along with comparison statistics for transplant program staffing benchmarks with the 2021 data.
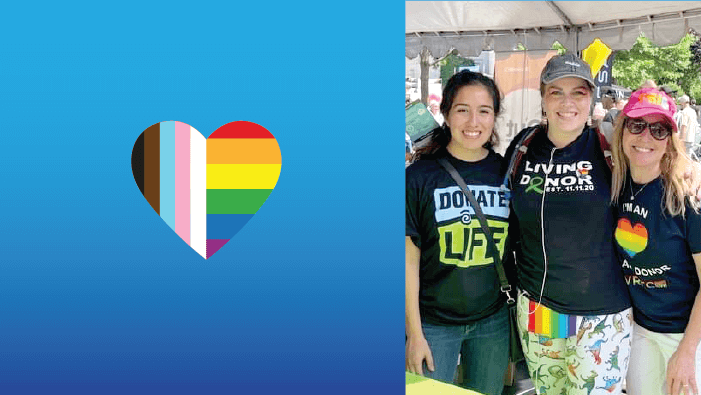
D.C. OPO’s LGBTQIA+ advocacy work increases organ donation.

The actions will increase the equity of access to liver transplant, and were among a number of additional measures adopted by the board.
A transition plan will allow race-neutral recalculation of certain candidates’ eGFR values and modifications to their waiting times.

The action, to take effect July 27, will ensure that all candidates are consistently assessed in an equitable fashion.

Shepard’s bold leadership set UNOS on a firm foundation to advance equity and save more lives in coming years.