
Implementation notice: Requirement for race-neutral eGFR formulas in effect
Transplant hospitals are able to take immediate action to benefit a select population of Black candidates impacted by race-inclusive eGFR calculations.

Transplant hospitals are able to take immediate action to benefit a select population of Black candidates impacted by race-inclusive eGFR calculations.

Four exclusion criteria in OPTN Policy 14.4.E have been updated to align with current research and guidelines as well as ensure consistency throughout OPTN policy language.

Report covers activity since the implementation of the acuity circles liver allocation policy.

New FDA announcements on Paxlovid and Novavax vaccine, Adjuvanted; Summer 2022 regional meetings to be in-person

Professional education on this policy change is now available in UNOS Connect. These policy changes take effect July 26.

On Sept. 27, UNOS will launch the first in a series of enhancements to the visual design and workflows in Waitlist.

New system will more holistically evaluate program performance.
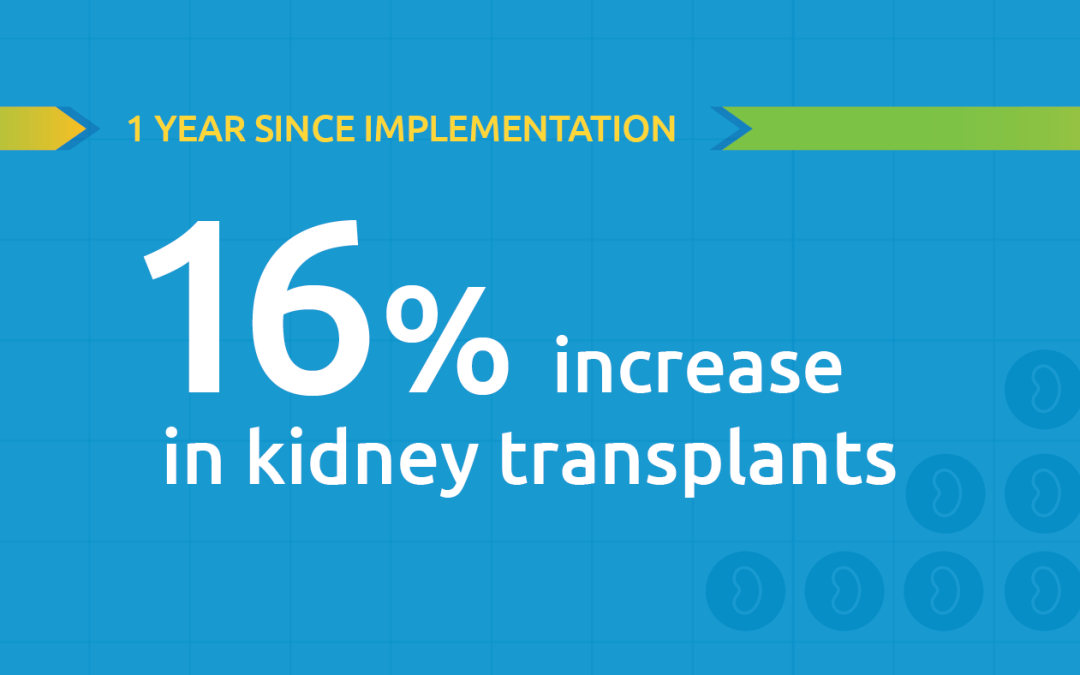
Aimed at increasing equity, a report on new allocation policy shows increases in transplant among key populations.

The F.M. Kirby Foundation Organ Center is celebrating its 40th year of continuous operation without any service interruptions

This new enhancement makes keeping up to date on essential education easier than ever.