in focus
Through the HOPE Act, 24 hospitals have performed a total of 223 transplants (170 kidney and 53 liver transplants).
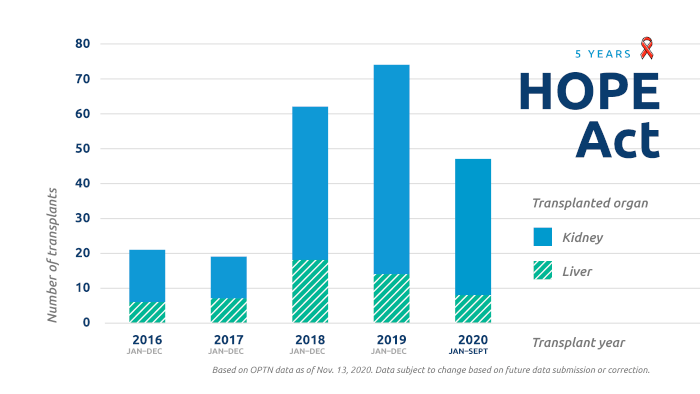
Five years after its implementation, the HIV Organ Policy Equity Act (also known as the HOPE Act) has continued to provide transplant opportunities for candidates with HIV who are willing to accept organ offers from donors identified as HIV-positive.
A total of 112 donors identified as HIV-positive have had at least one organ transplanted. Two kidney transplants were made possible through living organ donation.
The HOPE Act, signed into law November 21, 2013, called for the use of organs from HIV-positive donors for transplantation into HIV-positive candidates under approved research protocols designed to evaluate the feasibility, effectiveness and safety of such organ transplants.
The provisions of the Act took effect on November 21, 2015. Initially the protocol only authorized kidney and liver transplants. In a recent update, implemented in May 2020, organs of any type may now be transplanted under HOPE Act protocols.
As of November 2020, 30 transplant hospitals are enrolled with the OPTN to participate in HOPE Act research. Participating hospitals must conduct transplants under IRB-approved research protocols conforming to the Final Human Immunodeficiency Virus (HIV) Organ Policy Equity (HOPE) Act Safeguards and Research Criteria for Transplantation of Organs Infected with HIV, which were developed by the National Institute of Allergy and Infectious Diseases, one of the National Institutes of Health.
Organ procurement organizations are able to run matches for HIV-positive donors. The only candidates who will appear on match runs for these donor offers will be those listed at transplant programs that have an IRB-approved protocol, and whose HIV status and willingness to accept an HIV positive organ has been confirmed.
Learn more about the HOPE Act and related OPTN policy.
In focus
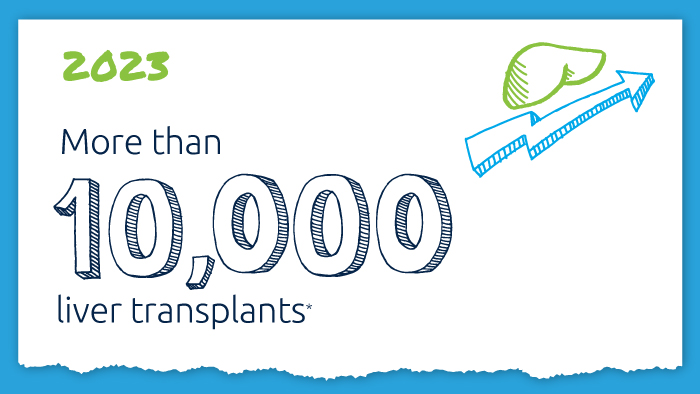
A decade of record increases in liver transplant
10,660 liver transplants, the most ever in a year.
Black kidney candidates are receiving waiting time modifications, helping them get the organs they need
Latest kidney monitoring report shows two new kidney polices are working as intended

Research in focus: examining organ offers
Three recent studies from UNOS researchers examine offer acceptance practices and impact of Offer Filters tool.
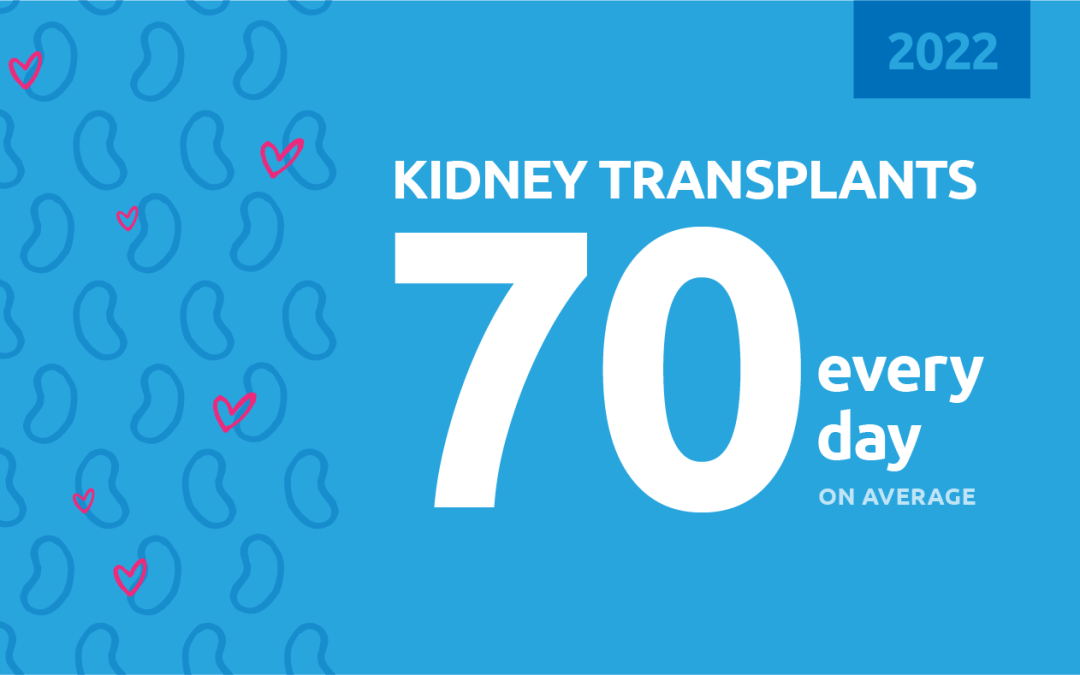
New milestone reached in kidney donation and transplant
For the first time, more than 25,000 kidney transplants were performed in a single year

