
Implementation notice: Phase I of new kidney medical urgency requirements
December 1, 2020
Kidney programs should update candidate data ahead of Dec. 15 final implementation.

Five years of HOPE Act; observations from Sander Florman, M.D.
November 20, 2020
Transplant program director at the hospital performing the most HOPE Act transplants reflects on its significance at the five-year milestone.

Innovating to strengthen the organ transplant system
October 30, 2020
UNOS Labs tries out new ideas in behavioral research, data science and technology. Our researchers develop innovative solutions to improve the national organ network and increase organ utilization.
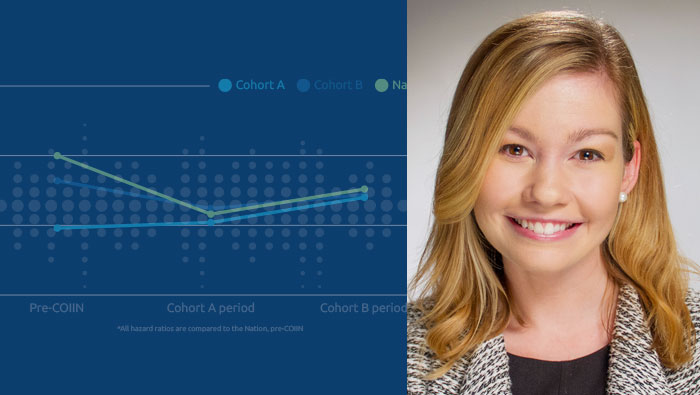
UNOS research analyst Julia Foutz on reducing the organ shortage and saving more lives
October 2, 2020
Research shows collaborative improvement has potential to increase kidney utilization.

UNOS receives $106,000 grant from Fresenius foundation
September 24, 2020
Fresenius Medical Care Foundation announces $106,000 grant to UNOS toward improving organ transportation, tracking and logistics

COVID-19 updates: Aug. 28, 2020
August 28, 2020
Special public comment proposal. Site surveys and OPTN committee meetings to be held virtually through Oct. 31. Plus, top COVID resources and webinars.
Page 49 of 68
