Audience:
- Transplant program directors and administrators
- Transplant program UNetSM users
- All member representatives and alternate representatives
Implementation date:
Jan. 11, 2022
At-a-glance
Effective immediately, OPTN Policy 1.4.F has been reinstated, addressing data submission requirements for transplant candidates in cases where data collection is affected by the COVID-19 outbreak. This is the same policy earlier in effect from March 17, 2020 to July 27, 2021. It authorizes transplant programs, as needed, to “carry forward” the most recent clinical data available when obtaining updated data is not feasible due to effects of the COVID-19 pandemic. Details of the policy are contained in the policy notice on the OPTN website.
Several transplant hospitals requested reinstatement of the policy due to resource challenges posed by the current surge in COVID-19 cases. The OPTN Executive Committee approved this action on Jan. 11, 2022, as an emergency policy per the OPTN bylaws. The policy will expire in 90 days unless the Executive Committee deems it necessary to continue in light of the current COVID-19 situation.
What you need to do
In all instances, continue to exercise your medical judgment in providing care for your transplant candidates. If issues relating to COVID-19 alter your usual decisions or actions, please document this in the candidate’s medical record.
For maintaining data to support a transplant candidate’s listing status or waiting time, please use the most updated clinical data you have available and make all reasonable efforts to meet OPTN requirements. If your program is unable to collect updated data due to issues related to COVID-19, or if in your medical judgment you opt not to collect updated data because of COVID-19 considerations, you may report the most recent clinical data values you previously submitted. In this instance, please report the date you are submitting the data as the actual date of the test and document these actions in the candidate’s medical record. The documentation must include the circumstances that support using the policy.
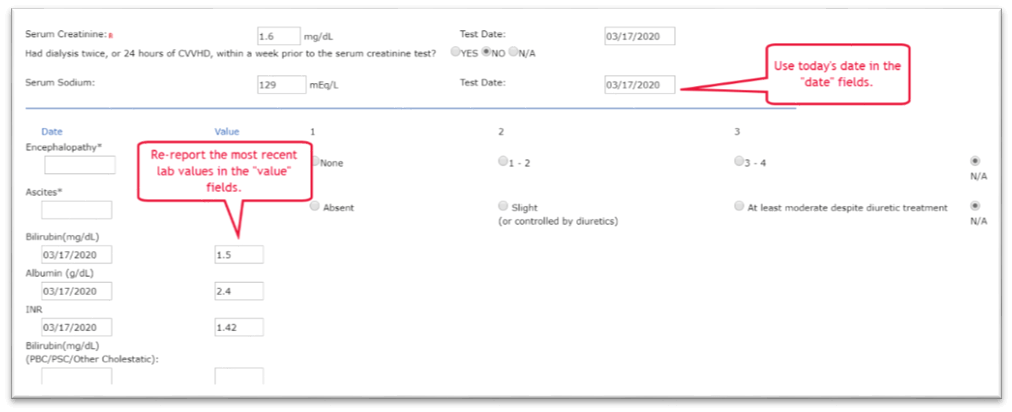
Illustrated example
Submission of new MELD lab values for a liver candidate to prevent downgrade due to an inability to obtain new labs as a result of COVID-19:
An example of acceptable documentation would be a note in the candidate’s medical record such as, “1/12/2022 – updated candidate record in Waitlist. Due to COVID-19 emergency actions, candidate’s previously reported clinical data was reported with today’s date.”
Questions?
If you have questions relating to implementation, contact UNOS Customer Service at 800-978-4334. For policy-related questions, send an e-mail to [email protected].